Achievements and Innovation
• The kinetics for a number of solid-phase reactions between metals and nonmetals occurring at high temperatures (up to 3000 oC) including strongly non-isothermal conditions was studied by applying a novel electrothermography method. It was shown that isothermal kinetic data not always are suitable for describing non-isothermal processes;
• Recently emphasis is given to the features of chemical reactions under non-isothermal conditions and the influence of heating rate on the course of chemical reactions. It was established for a number of heterogeneous systems that the heating rate has a decisive role in the direction of the reaction, its kinetic laws, and also on the microstructure of the formed solid products. It was shown that rapid heating of initial substances plays a crucial role also in providing favorable conditions for self-sustaining propagation for a number of reactions with the participation of solids;
• New mathematical models on reaction-diffusion with unified boundary conditions were developed with the possibility of computer visualization. For the first time solid-phase diffusion coefficients of carbon and silicon in a series of transition metals' carbides and silicides phases were determined within a wide temperature interval;
• A new approach was developed in the field of chemical stimulation of combustion processes in heterogeneous systems with the participation of solid active additives (combustion promoters). The approach includes 2 main aspects: thermal and kinetic. In the first case, the main factor contributing to the reaction proceeding under controlled combustion mode is ensuring of a certain thermal regime (Tad>Tad(min)). In the latter case, a high reaction rate is achieved by changing the mechanism of the overall reaction without a significant change in its thermal regime. In most cases, these two types of activation modes occur together and in this case we deal with so-called thermo-kinetic activation of reactions. On the basis of this approach, new technologies for up to 40 ceramic materials were developed. A criterion for searching for new combustion promoters was formulated and several such compounds (some inorganic salts, PTFE, melamine, polyvinylchloride, etc.) were offered, which were effectively applied for a number of systems.
INNOVATION ACTIVITY
• The laboratory has more than 40 accomplished technological developments for producing of powders of nonoxide ceramics, metal powders, ingots of metals and alloys, high-porous (foam-like) metals and ceramics under combustion mode.
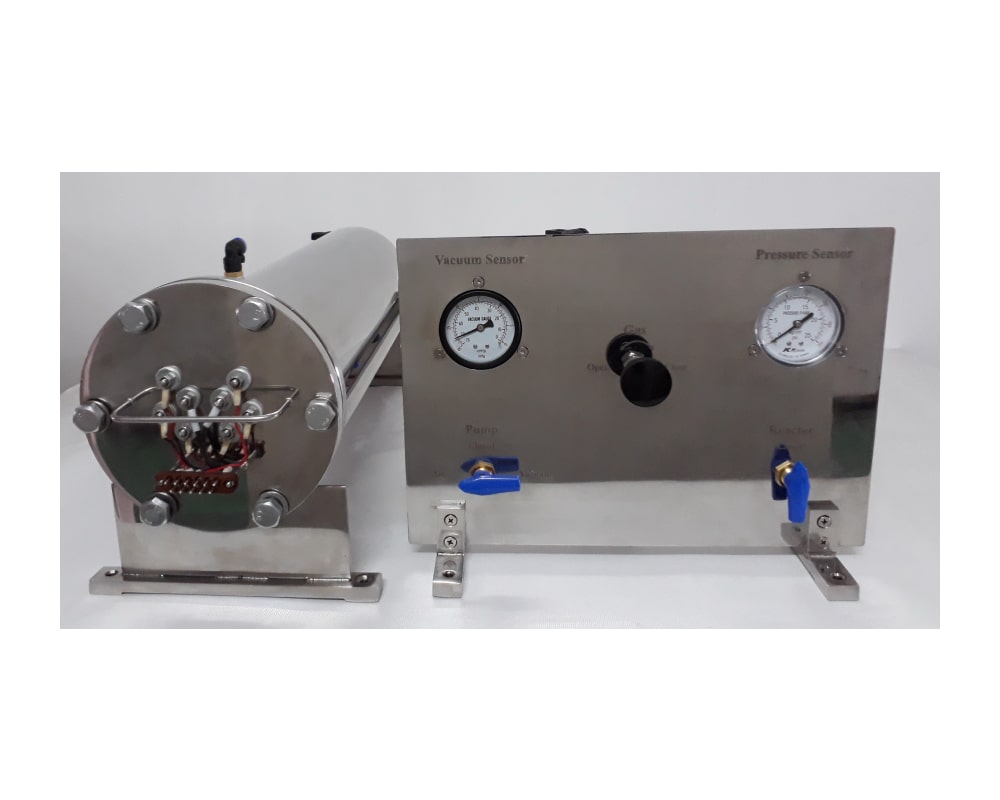
• An electrothermography setup with wide functional possibilities on electronic control and appropriate software were developed and constructed destined for studying the kinetics of solid-phase rapid chemical transformations under isothermal and non-isothermal conditions. Three generations of electrothermography apparatus were developed during the last 15 years.
• High-speed scanning electrothermography (HS-SET-3) setup for performing studies on metal wires with the possibility of controlled heating of substances with a rate up to 1 million K/s.
• High-speed temperature scanner (HSTS-2) for performing studies with powder mixtures with the possibility of controlled heating of substances with a rate up to 10000 K/min.
• Currently, the laboratory is developing new approaches to obtaining copper powder from various raw materials (concentrates, industrial wastes) based on the SHS process.